VitaMK7®
The bacteria in our gut cannot produce enough vitamin K2 alone – which is why nutraceuticals and functional foods and beverages are made with vitaMK7®. Natural Vitamin K2 is part of the Vitamin K family, a group of fat-soluble compounds having hemostatic, anti-osteoporotic, and antioxidant activities. vitaMK7® is the optimal vitamin K2 product, with more than 99% all-trans menaquinone-7 (MK7).
The result of a patented biotransformation process of Bacillus subtilis, vitaMK7®‘s production does not rely on other chemical compounds. MK7 is the only active form of vitamin K2.
vitaMK7® is known for regulating calcium in the body, keeping it from the cardiovascular system while driving it to the bones. From childhood to old age, vitaMK7® supports stronger bones throughout our lives.

- Product category
Active ingredients
- Market segments
-
Mobility & Joint
Reproduction & Women’s
Wellness & Immune
- Health benefits
-
Bone
 Cardiovascular
CardiovascularImmunity
 Menopause
Menopause Muscle
Muscle Sports Nutrition
Sports Nutrition
- Allergens and
certificates Gluten-free
Halal
Kosher
Lactose-free
Non GMO
 Vegetarian and Vegan
Vegetarian and Vegan
- Download
- Download the brochure
vitaMK7®: natural, clean, pure, and trustworthy
We are proud that both the source and the fermentation manufacturing process used to produce vitaMK7® are natural. Scientific experts commonly recognize this fermentation process as the only biotransformation process to deliver a natural vitamin K2. Due to its exceptional purity, vitaMK7® has an extraordinary shelf life (approximately four years at room temperature) and a short time light recovery (approximately 48 hours).
For these reasons, key brand owners in the vitamin industry favor vitaMK7®.
Embracing the clean label concept

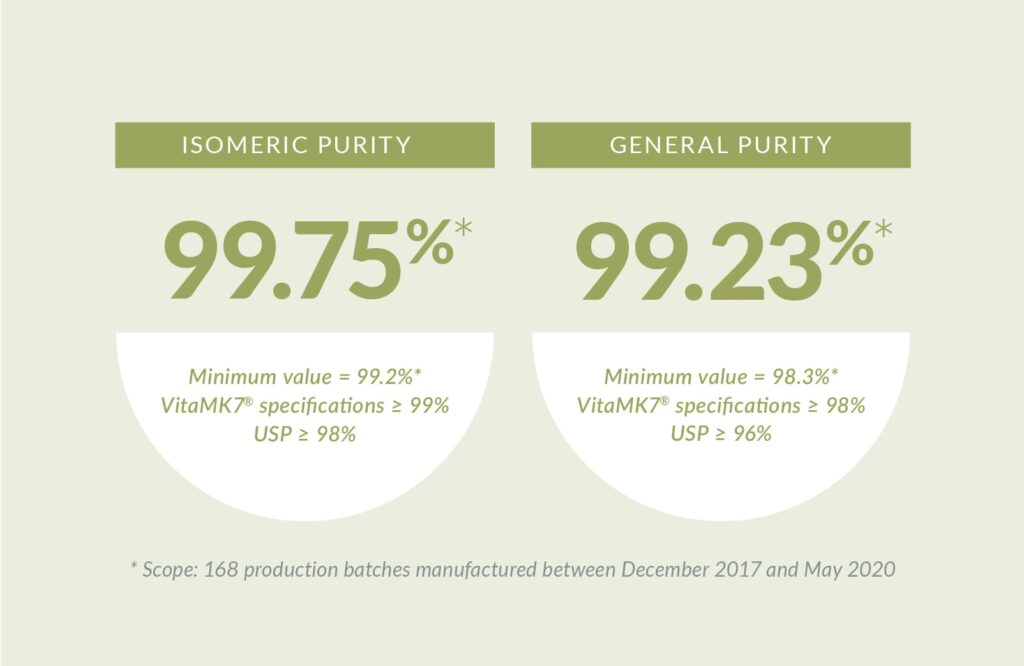
Achieving the highest level of purity
Trusted European manufacturing
Gnosis by Lesaffre is the sole European producer of Vitamin K2 as MK-7, where the entire supply chain, from the raw material to the finished product is without intermediates or brokers. The entire production process occurs in our cGMP-approved plant in compliance with the strictest quality rules and ISO and HACCP quality systems.

And more...
- Chemical & metal free
- Solvent free
- Published preclinical and clinical studies guaranteeing vitaMK7® efficacy
- Wide variety of powders and oil grades
- Patented natural vitamin K2: US Patent No. 7,718,407 / EU Patent No. 1 803 820 / JP Patent No. 5 043 425
- No additives and preservatives
- No need for calcium addition
- No synthetic process
- No bacterial residues
Related products
Tt enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Vitamin K2
Besides its proven role in bone and cardiovascular health, new research has linked K2 cardiovascular benefits, impacting new health areas and conditions. Vitamin K2 may be the missing link between diet and several chronic conditions. Explore our resources to learn more about the health benefits, science, and market applications of Vitamin K2.
Cardiovascular health
Consumers recognize they must be proactive about protecting their health, including shoring up their cardiovascular system to carry them into their senior years. They are seeking heart-healthy supplements that give them the opportunity to avoid pharmaceuticals, recognizing that obtaining proper nutrition can make a significant difference.
Frequently asked questions
Can’t find what you are looking for?
Latest news & events

- MenaQ7®
- Mobility & Joint Health
- News
- Nov 17, 2023
New Study Highlights Impact of Proactive Bone Health Strategies
New research shows increasing bone mineral density by 3% equates to a 45% reduction...

- MenaQ7®
- Mobility & Joint Health
- News
- Oct 23, 2023
Promoting Optimal Health in Korea with MenaQ7® and Quatrefolic®
MenaQ7® and Quatrefolic® for elderly health applications were highlighted during a seminar organized by...

- Emothion
- Lynside®
- MenaQ7®
- Sep 15, 2023
Join us at Vitafoods Asia booth G11
Gnosis by Lesaffre will be present at Vitafoods Asia this coming 20-22 September. Join...



